097 原子的電離能
Ionization Energy of Atoms
(https://www.nuclear-power.com/)
原子的電離能
Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.
X + energy → X+ + e−
where X is any atom or molecule capable of being ionized, X+ is that atom or molecule with an electron removed (positive ion), and e− is the removed electron.
電離能,又稱電離勢,是從中性原子中移去一個電子所必需的能量。
X+能量→X+ + e−
其中X是任何能夠被電離的原子或分子,X+是移除一個電子的原子或分子(正離子),e-是移除的電子。
There is an ionization energy for each successive electron removed. The electrons that circle the nucleus move in fairly well-defined orbits. Some of these electrons are more tightly bound in the atom than others. For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88,000 eV is required to remove the innermost electron.
每一個連續的電子移除都有一個電離能。環繞原子核的電子在相當明確的軌道上運動。有些電子在原子中的束縛比其他電子更緊密。例如,從鉛原子中移除最外層的電子只需要7.38 eV,而移除最內層的電子則需要88,000 eV。
- Ionization energy is lowest for the alkali metals which have a single electron outside a closed shell.
- 對於封閉殼層外只有一個電子的鹼金屬,電離能最低。
- Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells.
- 對於具有封閉殼層的惰性氣體,電離能沿週期最大值一行遞增。
For example, sodium requires only 496 kJ/mol or 5.14 eV/atom to ionize it. On the other hand neon, the noble gas, immediately preceding it in the periodic table, requires 2081 kJ/mol or 21.56 eV/atom.
例如,鈉離子僅需496 kJ/mol或5.14 eV/原子即可電離。另一方面,元素週期表中緊挨著它的惰性氣體氖,需要2081 kJ/mol或21.56 eV/原子。
The ionization energy associated with removal of the first electron is most commonly used. The nth ionization energy refers to the amount of energy required to remove an electron from the species with a charge of (n-1).
與移除第一個電子有關的電離能是最常用的。第n次電離能是指從帶(n-1)電荷的物質中移去一個電子所需的能量。
1st ionization energy
第一電離能
X → X+ + e−
2nd ionization energy
第二電離能
X+ → X2+ + e−
3rd ionization energy
第三電離能
X2+ → X3+ + e−
For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88,000 eV is required to remove the innermost electron.
例如,從鉛原子中移除最外層的電子只需要7.38 eV,而移除最內層的電子則需要88,000 eV。
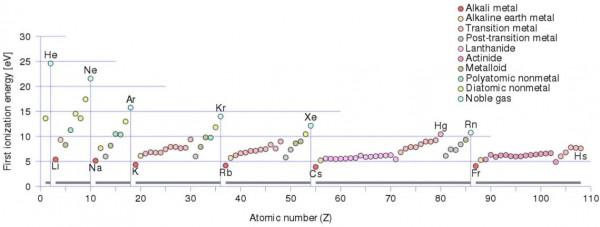
來源: wikipedia.org License: CC BY-SA 3.0
(待續)