089 原子的玻爾模型
Bohr Model of Atom
(https://www.nuclear-power.com/)
原子的玻爾模型
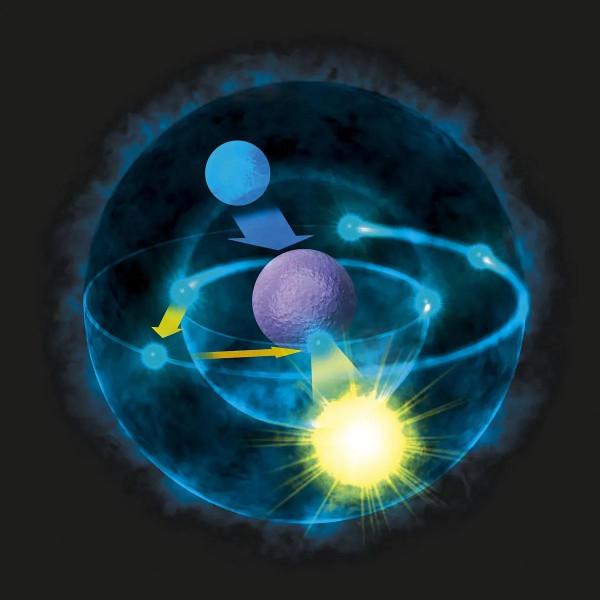
“In atomic physics, the Bohr model if the atom (also known as the Rutherford-Bohr model) is modern model of the hydrogen atom introduced by Danish physicist Niels Bohr. All features of Bohr model of the atom can be summarized in Bohr’s Postulates. The Bohr model adopted Planck’s quantum hypothesis and he proposed a model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits.”
“在原子物理學中,原子玻爾模型(又稱盧瑟福-玻爾模型)是丹麥物理學家尼爾斯·玻爾提出的氫原子現代模型。玻爾原子模型的所有特徵都可以概括在玻爾公設中。波爾模型採用了普朗克的量子假設,他提出了一個模型,假設原子中的電子繞原子核執行,但只能在限定的軌道中執行。”
In atomic physics, the Bohr model if the atom (also known as the Rutherford-Bohr model) is modern model of the hydrogen atom introduced by Danish physicist Niels Bohr working with Ernest Rutherford at the University of Manchester in 1913.
在原子物理學中,玻爾原子模型(也被稱為盧瑟福-玻爾模型)是1913年由丹麥物理學家尼爾斯·玻爾和歐內斯特·盧瑟福在曼徹斯特大學合作提出的氫原子現代模型。
This model provides especially the solution to the problem of the failure of classical physics in the field of atomic physics. Classical electromagnetic theory, makes three entirely wrong predictions about atoms:
- atoms should emit light continuously,
- atoms should be unstable,
- the light they emit should have a continuous spectrum.
該模型特別解決了經典物理學在原子物理學領域的失效問題。經典的電磁理論,對原子做出了三個完全錯誤的預測:
- 原子應該不斷地發出光,
- 原子應該不穩定,
- 其發出的光應有一個連續的光譜。
注:
(以下內容來自百度百科)
玻爾模型
盧瑟福的理論吸引了一位來自丹麥的年輕人,他的名字叫尼爾斯·玻爾(Niels Bohr,1885-1962),在盧瑟福模型的基礎上,他提出了電子在核外的量子化軌道,解決了原子結構的穩定性問題,描繪出了完整而令人信服的原子結構學說。
(待續)